Corrosion of high carbon steel strand can be a serious problem in long term civil engineering applications. In mining, however, the incidences of cablebolt corrosion causing serious problems are rare. This is due primarily to the short time frame involved in open stope support in underground mining.
Corrosion problems observed by the authors in mining environments were typically in long term support in open pits where the groundwater was acidic or saline and in long term support in underground sulphide deposits. Cut and fill applications in wet conditions where fractured stope backs could remain (supported) for up to a year were notably susceptible to corrosion. Serious failure, due to corrosion and rupture of the strand, can occur in such applications.
The nature of corrosion is extremely complex and a fundamental discussion is beyond the scope of this book. It is the intent here to discuss some of the important factors involved in corrosion so that the engineer may assess the potential for problematic corrosion and take steps to prevent it or make the appropriate design allowances for it.
Most common refined metals are inherently unstable ionic materials composed of arrays of single atoms which possess a full compliment of electrons. Metals such as iron normally tend to give up electrons at room temperature (gold is a notable exception) and become involved in reactions leading to the formation of more stable compounds such as iron oxide or iron hydroxide (rust). The release of electrons is termed an anodic reaction and the acceptance of electrons a cathodic reaction. Both reactions must occur for corrosion to take place. Since metals such as the iron found in steel cable are normally willing to give up their electrons, it is normally the presence of a cathode which determines the corrosion potential.
The cathodic reaction (involving the consumption of electrons released anodically from the iron) can be made possible by the presence of an acid, sulphate, water and/or oxygen.
Corrosion of steel (iron) can be divided into four basic categories (Illston et al., 1979; Pohlman, 1987):
– Dry corrosion
– Wet corrosion
– Corrosion of immersed metals and alloys Induced or accelerated corrosion (includes influence of stress)
The following discussion is confined to corrosion of cablebolts and as such is incomplete as a comprehensive examination of general corrosion.
Dry Corrosion
Dry corrosion is an inevitable consequence of medium- to long-term storage of cablebolts in even the most ideal conditions. It involves the formation of iron oxide (Fe0) as iron atoms combine with atmospheric oxygen. Once the process initiates on a clean surface, it spreads fairly rapidly to involve most of the exposed surface. While Fe0 forms an adherent film on steel surfaces and can actually form an impervious layer, it can be vulnerable to cracking and as such fresh iron is constantly being exposed and the process continues. In the perspective of cablebolting in mining, however, dry oxidation is a relatively slow chemical process and is of only minor consequence. Light surface (dry) corrosion has been shown (Goris, 1990) to improve bond performance of cablebolts by up to 20% in ideal conditions, although deliberate rusting of cablebolts is not advocated by the authors. The process is accelerated by higher surface temperatures (e.g. if the cables are exposed daily, over long periods, to direct and intense sunlight).
Heavy surface rust on newly shipped cables is usually the result of exposure to moisture and subsequent atmospheric corrosion which can be very detrimental to the performance of the cablebolts.
Wet or Atmospheric Corrosion
In a wet or humid environment, the corrosion process is accelerated and can involve a wider variety of cathodic reactions. Water and oxygen become jointly involved in the cathodic reaction and result in other compounds such as 2Fe(OH) ,3Fe O (magnetite), or Fe O (hema 3 4 2 3 tite). These compounds are much less adhesive then FeO and less likely to form a self-arresting film.
Corrosion products formed on cablebolts by wet corrosion are more likely to have a greasy feel as compared to the dry, rough texture of FeO film and are more likely to be associated with other film substances such as oils and additional moisture. These products are likely to have a detrimental effect on bond capacity of cablebolts. Clearly, unchecked corrosion reduces the cross-sectional area of steel in the cable and ultimately reduces the tensile capacity of the steel to unacceptable levels. Ductility and displacement capacity is also reduced (embrittlement).
The presence of water on the surface of the cablebolt also increases the potential for galvanic corrosion. The same wet corrosion cathodic reactions occur, accelerated by the presence of an electrolyte such as chloride, sulphate or hydroxide. Without electrolytes in a static solution, the corrosion process is self-limiting. Iron ions (e.g. Fe ) move into solution adjacent to the steel surface 2+ leaving behind free electrons (2e ) in the steel solid. The concentration of iron ions -in solution and free electrons in the steel creates an electrical potential difference which resists further dissolution of iron ions.
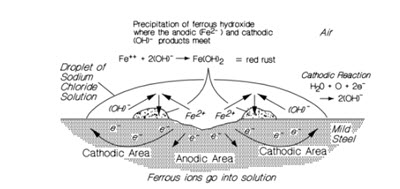
The effects of electrolytes in the surface water is best illustrated in the above example. A drop of water on the surface of the steel contains a dissolved electrolyte such as sodium chloride (which forms a solution of free sodium, Na ,+ and chloride, Cl , ions). The presence of electrolytes permits the transport of iron – ions as FeCl away from the corrosion (anode) site at the centre of the drop. At the same time, water and oxygen combine at the perimeter of the drop with the free electrons from the steel to form hydroxide ions (OH ) balanced by Na in solution. – + These move in the opposite direction to the FeCl generating a current (electron flow) in the steel supplying electrons to the drop perimeter as more iron ions go into the solution at the drop centre. Between the active centre (anode) and the drop perimeter (cathode) the iron ions combine with the hydroxide to form ferrous hydroxide.
This in turn becomes a relatively stable and complex hydrated oxide known as rust. The sodium and chloride transport ions are freed to carry on the process. The cyclic nature of the process combined with the fact that the corrosion product (rust) is not deposited at the anode (as it is with dry corrosion) means that this form of galvanic corrosion is not self-limiting and can be very aggressive. This is particularly true in mining environments given the high concentration of chloride and sulphate ions in mine waters (Minick and Olson, 1987).
Moist corrosion is particularly enhanced by crevices such as those formed by the flutes of a cable. Crevices are particularly good at retaining moisture and the conditions are perfect for differential aeration with low oxygen supply at the tip of the crevice compared with the rest of the cable. If a weak electrolyte is present, an aggressive corrosion cell is thus generated. This corrosion is particularly detrimental as the corrosion product (rust) readily fills the flutes of the cable preventing the penetration of grout and seriously reducing the cable/grout interlock essential for cable bond strength.